- Number Of Protons In An Atom Quizlet
- Atoms Protons Neutrons Electrons Worksheet
- Periodic Table Of Elements Protons Neutrons
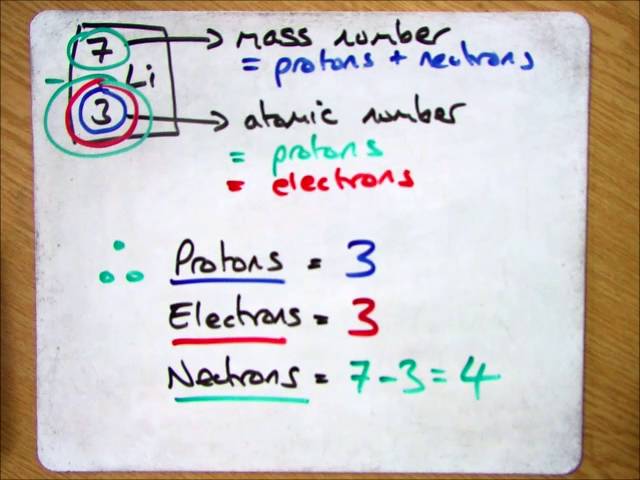
The atomic number is the number of protons in an atom of an element. In our example, krypton's atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus. The interesting thing here is that every atom of krypton contains 36 protons. Finding the number of protons, electrons and neutrons in an atom. You can find the number of protons in an atom easily because it is just the atomic number of the atom. If you have an aluminium atom, it will have 13 protons in each atom because its atomic number is 13. Quarkxpress 2016 for mac torrent. Likewise, gold has atomic number 79, so each gold atom has 79 protons in it.
Answer: atomic
Most relevant text from all around the web:

The number of protons in at atom is its ________ number. The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom the atomic number is also equal to the number of electrons. The sum of the atomic number Z and the number of neutrons N gives the mass numberA of an atom. Sin… The number of protons in an atom is called its atomic number . Ernest Rutherford (1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom … Atom - Wikipedia Mass number - Wikipedia Atomic number - Wikipedia Atomic number - Wikipedia In chemistry the number of protons in the nucleus of an atom is known as the atomic number which determines the chemical element to which the atom belongs. For example the atomic number of chlorine is 17; this means that each chlorine atom has 17 protons and that all atoms with 17 protons are chlorine atoms. The chemical properties of each atom are determined by the number of (negatively charged) electrons which for neutral atoms is equal to the number of (positive) protons so that the total charge i… All atoms above atomic number 82 (82 protons lead) are radioactive. There are three main types of radioactive decay; alpha beta and gamma. Alpha decay is when the atom shoots out a particle having two protons and two neutrons. This is essentially a helium nucleus. mass number (symbol: A) which is the sum of the number of protons and number of neutrons in the nucleus of an atom relative atomic mass (also called atomic weight ; symbol: A r ) which is the ratio of the average mass per atom of ..


/GettyImages-577639404-3651e7c556f24804b658f4687a6aa46b.jpg)
Number Of Protons In An Atom Quizlet
Disclaimer:
Atoms Protons Neutrons Electrons Worksheet
Our tool is still learning and trying its best to find the correct answer to your question. Now its your turn, 'The more we share The more we have'. Comment any other details to improve the description, we will update answer while you visit us next time..Kindly check our comments section, Sometimes our tool may wrong but not our users.
Periodic Table Of Elements Protons Neutrons
Are We Wrong To Think We're Right? Then Give Right Answer Below As Comment
