- Atoms And Elements Worksheet Pdf
- Atoms And Elements Ppt
- Difference Between Elements And Atoms
- Atoms And Elements Notes
- Atoms And Elements Video
- Atoms And Elements Facts
An atom is the smallest unit of matter that retains all of the chemical properties of an element. For example, a gold coin is simply a very large number of gold atoms molded into the shape of a coin, with small amounts of other, contaminating elements. Atoms of the same element that differ in their numbers of neutrons are called isotopes. Many isotopes occur naturally. 4.9: Atomic Mass: The Average Mass of an Element’s Atoms In chemistry we very rarely deal with only one isotope of an element. We use a mixture of the isotopes of an element in chemical reactions and other aspects of. If an element comes from steel, it means it will contain steel atoms only. Therefore, to be precise, atoms are the smallest part or amounts of elements. This is the primary difference between an atom and element. Atoms are the simplest unit of a matter. In their center, atoms have a closely packed nucleus. Elements and Atomic Numbers Atoms of the same type make up elements. These elements are identified by the number of protons inside atomic nucleus. The number of protons an atom pos-sesses is called its atomic number. For instance, the element hydrogen has 1 proton in its nucleus, so its atomic num-ber is 1. Atoms are the basic building blocks of everything around us. They come in different kinds, called elements, but each atom shares certain characteristics in common. All atoms have a dense central core called the atomic nucleus.
By the end of this section, you will be able to:
- Discuss the relationships between matter, mass, elements, compounds, atoms, and subatomic particles
- Distinguish between atomic number and mass number
- Identify the key distinction between isotopes of the same element
- Explain how electrons occupy electron shells and their contribution to an atom’s relative stability
Introduction
The substance of the universe—from a grain of sand to a star—is called matter. Scientists define matter as anything that occupies space and has mass. An object’s mass and its weight are related concepts, but not quite the same. An object’s mass is the amount of matter contained in the object, and the object’s mass is the same whether that object is on Earth or in the zero-gravity environment of outer space. An object’s weight, on the other hand, is its mass as affected by the pull of gravity. Where gravity strongly pulls on an object’s mass its weight is greater than it is where gravity is less strong. An object of a certain mass weighs less on the moon, for example, than it does on Earth because the gravity of the moon is less than that of Earth. In other words, weight is variable, and is influenced by gravity. A piece of cheese that weighs a pound on Earth weighs only a few ounces on the moon.
Elements and Compounds
Atoms And Elements Worksheet Pdf
All matter in the natural world is composed of one or more of the 92 fundamental substances called elements. An element is a pure substance that is distinguished from all other matter by the fact that it cannot be created or broken down by ordinary chemical means. While your body can assemble many of the chemical compounds needed for life from their constituent elements, it cannot make elements. They must come from the environment. A familiar example of an element that you must take in is calcium (Ca++). Calcium is essential to the human body; it is absorbed and used for a number of processes, including strengthening bones. When you consume dairy products your digestive system breaks down the food into components small enough to cross into the bloodstream. Among these is calcium, which, because it is an element, cannot be broken down further. The elemental calcium in cheese, therefore, is the same as the calcium that forms your bones. Some other elements you might be familiar with are oxygen, sodium, and iron. The elements in the human body are shown in Figure 1, beginning with the most abundant: oxygen (O), carbon (C), hydrogen (H), and nitrogen (N). Each element’s name can be replaced by a one- or two-letter symbol; you will become familiar with some of these during this course. All the elements in your body are derived from the foods you eat and the air you breathe.
Atoms And Elements Ppt
Elements and Atoms: Atoms
Difference Between Elements And Atoms
By the end of the 18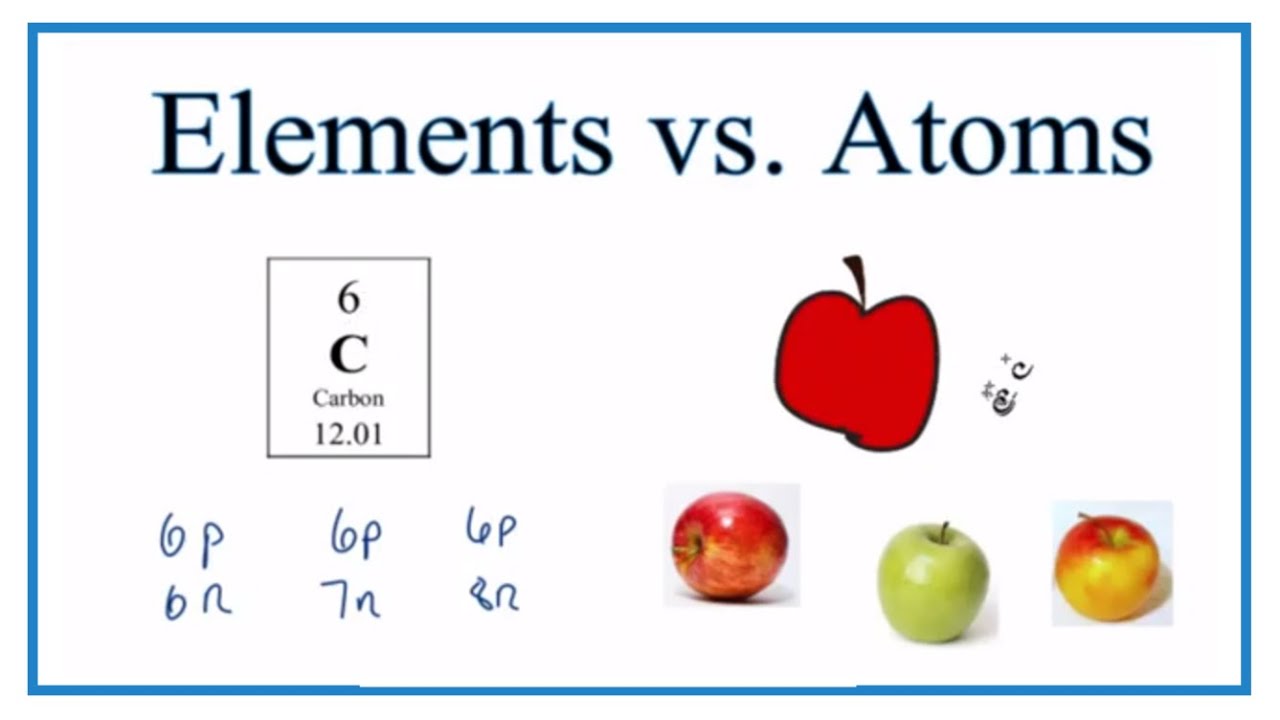 th century, the classical definition of an element as an ultimate product of chemical analysis had become established. This purely operational and macroscopic definition is independent of any theory concerning the nature of matter. By contrast, the modern definition of element is couched in terms of a discrete theory of matter, in terms of atoms. The same reference works consulted on the definition of element all agree when it comes to atom: an atom is the individual structure that constitutes the basic unit of any chemical element.
th century, the classical definition of an element as an ultimate product of chemical analysis had become established. This purely operational and macroscopic definition is independent of any theory concerning the nature of matter. By contrast, the modern definition of element is couched in terms of a discrete theory of matter, in terms of atoms. The same reference works consulted on the definition of element all agree when it comes to atom: an atom is the individual structure that constitutes the basic unit of any chemical element.The term atom has a long history. The concept has evolved from that of an indivisible ultimate particle of matter to a composite structure whose constituent parts themselves have constituent parts. The notion that materials come in discrete packets can be traced to the ancient Greeks. This classical atomism is most frequently associated with Leucippus and Democritus, and it was transmitted to later cultures throught the didactic poem De Rerum Natura by the Roman Lucretius. The atoms of the ancients were literally indivisible (the etymological meaning of atom from the Greek α (not) + τεμνω (cut). The atoms of Newton, so influential in the subsequent development of the concept, were also indestructible, structureless particles [Newton 1704].
The selections of this section will trace only a small part of the story of the atom, focusing on Dalton's hypothesis regarding the chemical significance of atoms and his program for determining one of their salient characteristics (their relative masses). The interaction between Dalton's hypothesis, the subsequent work of Gay-Lussac on combining volumes, and the insights of Avogadro form, in retrospect, a coherent whole, but illustrate, among other things, that progress in science is often non-linear.
This chapter's final selection foreshadows the book's final section, which examines some of the evidence that the atom was not in fact indivisible. Prout's hypotheses were a part of the early 19th-century concept of the atom, but were finally settled only in the first half of the 20th century. In the meantime, the concept of atom developed along distinct chemical and physical lines. 'Physical' atoms were a part of theories of matter: were these ultimate particles centers of force, vortices, packets of electrodynamic energy, or something else? In this respect, physicists were ready to look into the structure of the atom before chemists. At the same time, physical manifestations of the discrete nature of matter led to 'molecular' theories such as the kinetic theory of gases. Chemists were interested in the units of elements that entered into chemical combinations, often without regard for whether those units were or were not ultimate particles.
Numerous treatments of the history of atoms, of which I mention just a few, consider these and other aspects of the story. Pullman 1998 contains an overarching history of the concept of atom. Van Melsen 1952 concentrates on philosophical and pre-scientific facets of atomism. Rocke 1984 focuses on the chemical concept of atom in the 19th century. Knight 1967 treats scientific concepts of the atom, particularly in the 19th century. Fifa 16 for mac os. Outlook for mac мы mail. Knight 1968 contains facsimiles of many of the original papers mentioned in Knight 1967. Boorse & Motz 1966 is another source of original material and some commentary on the development of scientific atomism.
Atoms And Elements Notes
References
- Henry A. Boorse & Lloyd Motz, eds., The World of the Atom (New York: Basic Books, 1966), 2 vol.
- David M. Knight, Atoms and Elements (London: Hutchinson, 1967)
- David M. Knight, ed., Classical Scientific Papers: Chemistry (New York: American Elsevier, 1968)
- Titus Lucretius Carus, De Rerum Natura translated by William Ellery Leonard as Of the nature of things : a metrical translation (London: Dent, 1921)
- Andrew G. van Melsen, From Atomos to Atom (Pittsburgh: Duquene, 1952)
- Isaac Newton, Opticks (London, 1704), query 31
- Bernard Pullman, The Atom in the History of Human Thought (New York : Oxford University Press, 1998)
- Alan J. Rocke, Chemical Atomism in the Nineteenth Century: from Dalton to Cannizzaro (Columbus, OH: Ohio State, 1984)
Atoms And Elements Video
Back to the top of Classic Chemistry.Atoms And Elements Facts
